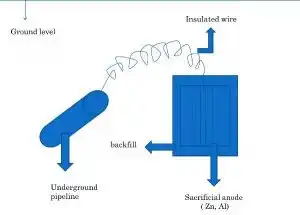
Sacrificial Anode
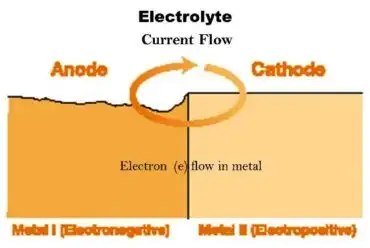
In the usual application, a galvanic anode, a piece of a more electrochemically “active” metal, is attached to the vulnerable metal surface where it is exposed to the corrosive liquid. Galvanic anodes are designed and selected to have a more “active” voltage (more negative electrochemical potential) than the metal of the target structure (typically steel). For effective CP, the potential of the steel surface is polarized (pushed) more negative until the surface has a uniform potential.
Metals like (Zn,Al,Mg) are used for making anode because they have very low electrochemical potential as compared to steel hence more ‘active’.
These metals act as anode and get corroded. For this purpose of increasing electrical contact, the active metal is placed in back fill (coal and NaCl). When it is consumed completely then replaced by a newer one.





No Comments