The Principles of Cathodic Protection
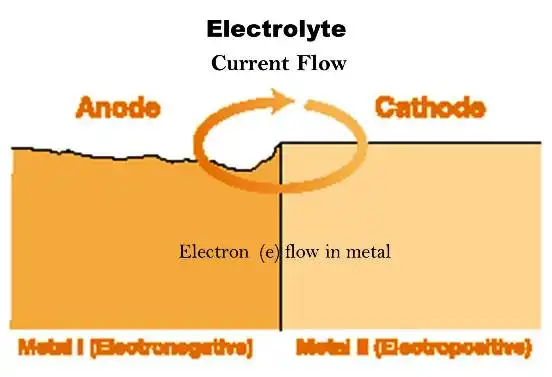
Metal that has been extracted from its primary ore (metal oxides or other free radicals) has a natural tendency to revert to that state under the action of oxygen and water. This action is called corrosion and the most common example is the rusting of steel. Corrosion is an electro-chemical system that comes to the […]
Cathodic Protection for Asset Protection from Cathpro Indonesia
 Cathpro Indonesia is an impressed current, flexible cable anode for use in cathodic protection systems for buried structures. Placed alongside a pipe or other buried metal structure, Cathpro Indonesia provides uniform cathodic protection to every point, with a minimum of interference from adjacent structures. What is Cathpro Indonesia? ... a Cable Anode Cathpro Indonesia [...]
Cathpro Indonesia is an impressed current, flexible cable anode for use in cathodic protection systems for buried structures. Placed alongside a pipe or other buried metal structure, Cathpro Indonesia provides uniform cathodic protection to every point, with a minimum of interference from adjacent structures. What is Cathpro Indonesia? ... a Cable Anode Cathpro Indonesia [...] Cathodic protection glossary
 Acid Containing an excess of hydrogen ions over hydroxyl ions. Alkaline Containing an excess of hydroxyl ions over hydrogen ions. Anode The electrode thru which direct cutting-edge enters an electrolyte. Anodic area The part of the steel surface that acts as an anode. Bond a chunk of metallic conductor, both strong or bendy, usually of [...]
Acid Containing an excess of hydrogen ions over hydroxyl ions. Alkaline Containing an excess of hydroxyl ions over hydrogen ions. Anode The electrode thru which direct cutting-edge enters an electrolyte. Anodic area The part of the steel surface that acts as an anode. Bond a chunk of metallic conductor, both strong or bendy, usually of [...] [:en]Cathodic Protection Material[:]

[:en][:]
Cathodic Protection Indonesia
Cathodic protection Indonesia is a method of protecting metal surfaces from corrosion. In this system, the protected metal acts as a cathode in an electrochemical cell. This system will turn the anodic part of the metal surface into cathode by delivering free electrons (electrical current) on the sacrificial method. The free electrons or electrical current [...][:en]Sacrificial Anode[:]

[:en]In the usual application, a galvanic anode, a piece of a more electrochemically “active” metal, is attached to the vulnerable metal surface where it is exposed to the corrosive liquid. Galvanic anodes are designed and selected to have a more “active” voltage (more negative electrochemical potential) than the metal of the target structure (typically steel). For effective […]
[:en]What is Cathodic protection?[:]

[:en][:]
