The Principles of Cathodic Protection
Metal that has been extracted from its primary ore (metal oxides or other free radicals) has a natural tendency to revert to that state under the action of oxygen and water. This action is called corrosion and the most common example is the rusting of steel.
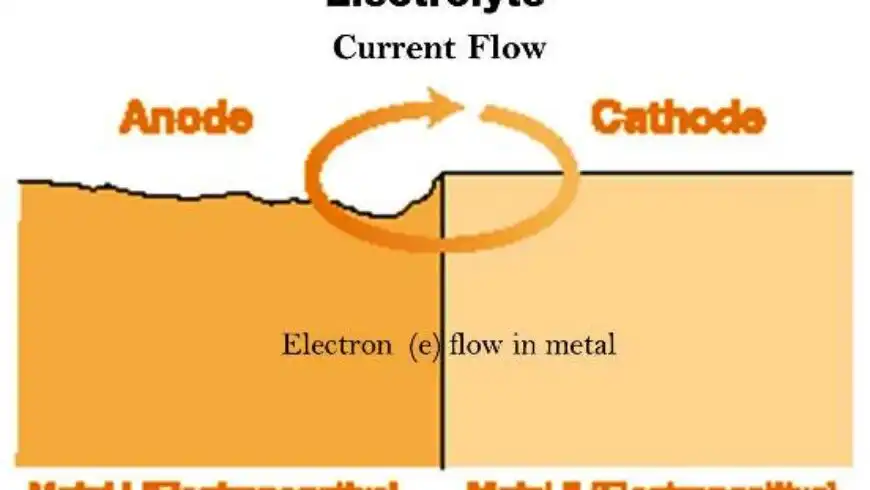
Corrosion is an electro-chemical system that comes to the passage of electric currents on a micro or macro scale. The change from the metallic to the combined form occurs by an “anodic” reaction:
M → M+ + e-
(metal) (soluble salt) (electron)
A common example is:
Fe → Fe++ + 2e-
This reaction produces free electrons, which pass within the metal to another site on the metal surface (the cathode), where it is consumed by the cathodic reaction. In acid solutions the cathodic reaction is:
2H+ + 2e- → H2
(hydrogen ions in solution) (gas)
In neutral solutions the cathodic reaction involves the
consumption of oxygen dissolved in the solution:
O2 + 2H2O + 4e- → 4OH-
. . (alkali)
Corrosion thus occurs at the anode but not at the cathode (unless the metal of the cathode is attacked by alkali).
Figure 1. Corrosion cell / Bimetallic corrosion
The anode and cathode in a corrosion process may be on two different metals connected together forming a bimetallic couple, or, as with rusting of steel, they may be close together on the same metal surface.
This corrosion process is initially caused by:
Differerence in natural potential in galvanic (bimetallic) couples. Metallurgical diversifications in the state of the metallic at various facets on the surface.
Local differences in the environment, such as variations in the supply of oxygen at the surface (oxygen rich areas become the cathode and oxygen depleted areas become the anode).
The precept of cathodic protection is in connecting an exterior anode to the metallic to be included and the passing of an electric dc present so that all locations of the steel floor turn into cathodic and hence do not corrode. The external anode may be a galvanic anode, where the current is a result of the potential difference between the two metals, or it may be an impressed current anode, where the current is impressed from an external dc power source. In electro-chemical terms, the electrical potential between the metal and the electrolyte solution with which it is in contact is made more negative, by the supply of negative charged electrons, to a value at which the corroding (anodic) reactions are stifled and only cathodic reactions can take place. In the discussion that follows it is assumed that the metal to be protected is carbon steel, which is the most common material used in construction. The cathodic protection of reinforcing carbon steel in reinforced concrete structures can be applied in a similar manner.
Cathodic protection can be achieved in two ways:
- by the use of galvanic (sacrificial) anodes, or
- by “impressed” current.
Galvanic anode systems employ reactive metals as auxiliary anodes that are directly electrically connected to the steel to be protected. The difference in natural potentials between the anode and the steel, as indicated by their relative positions in the electro-chemical series, causes a positive current to flow in the electrolyte, from the anode to the steel. Thus, the whole floor of the metallic turns into more negatively charged and turns into the cathode. The metals commonly used, as sacrificial anodes are aluminium, zinc and magnesium. These metals are alloyed to improve the long-term performance and dissolution characteristics.
Impressed-current systems employ inert (zero or low dissolution) anodes and use an external source of dc power (rectified ac) to impress a current from an external anode onto the cathode surface.
The connections are similar for the application of cathodic protection to metallic storage tanks, jetties, offshore structures and reinforced concrete structures.





No Comments